Study Shows Omnipod® 5 Improved Glycemic Measures for Patients with Type 1 Diabetes


Omnipod 5 is the first and only tubeless automated insulin delivery (AID) system in the US. A single-arm, multicenter, prospective study by Brown et al. evaluated the safety and effectiveness of Omnipod 5 and found it was safe and led to improved glycemic measures in the participants.1
The study had two phases, consisting of a 2 week standard therapy (ST) phase, followed by 3 months on the Omnipod 5 AID system. Out of 240 enrolled participants, 235 completed the study (111 children, age 6-13.9 and 124 adults/adolescents, age 14-70). All participants had been diagnosed with type 1 diabetes for 6 months or longer and had HbA1C lower than 10%. Participants also did not have any restrictions regarding eating or exercising during the study.
The primary endpoints for safety were incidence of severe hypoglycemia and diabetic ketoacidosis. The primary endpoints for effectiveness were change in HbA1c and time in range (TIR), defined as the percent of time spent in a sensor glucose range between 70–180 mg/dL.
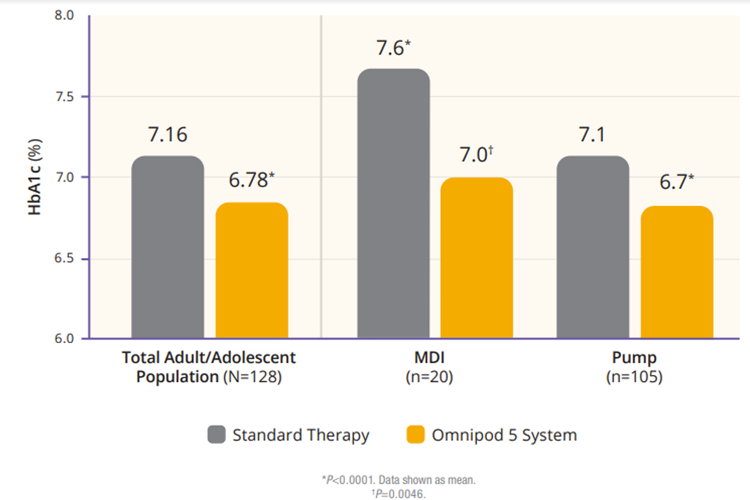
The authors analyzed the adult/adolescent cohort and the children cohort separately. The adult/adolescent cohort had a significant improvement in HbA1C. On standard therapy 45% of participants had an HbA1C below 7% and after 3 months of Omnipod 5, 66% achieved that, as shown in Figure 1.

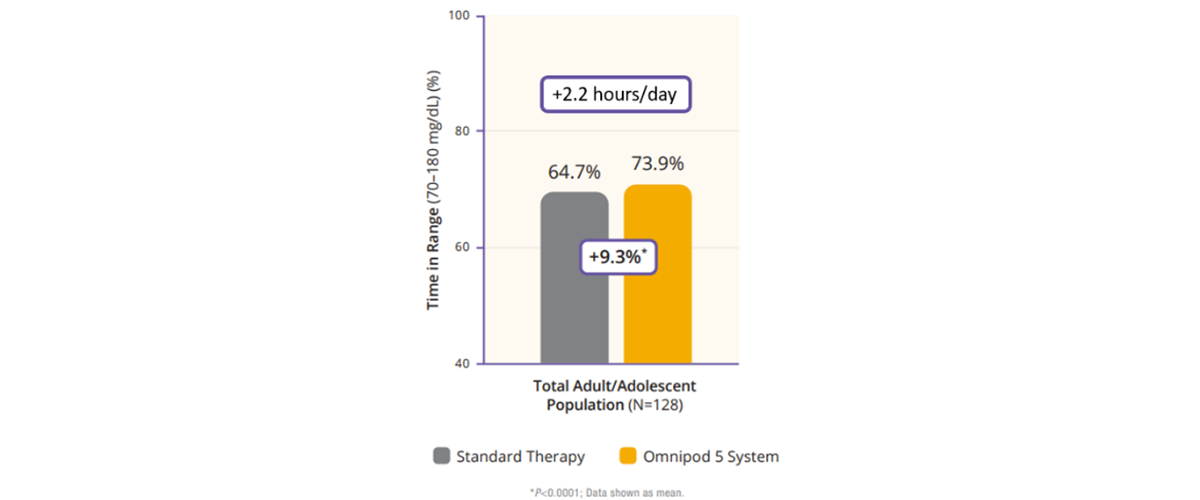
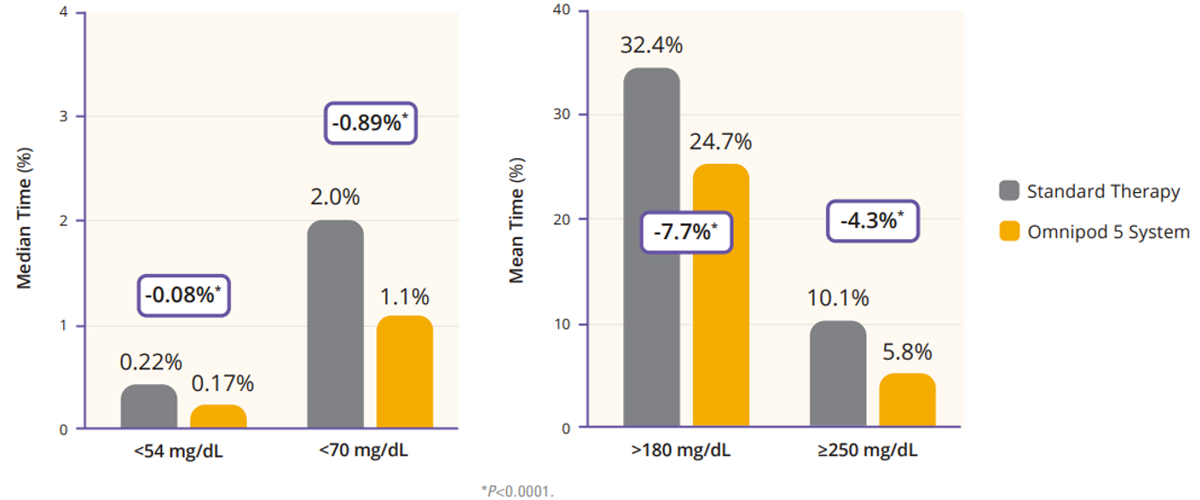
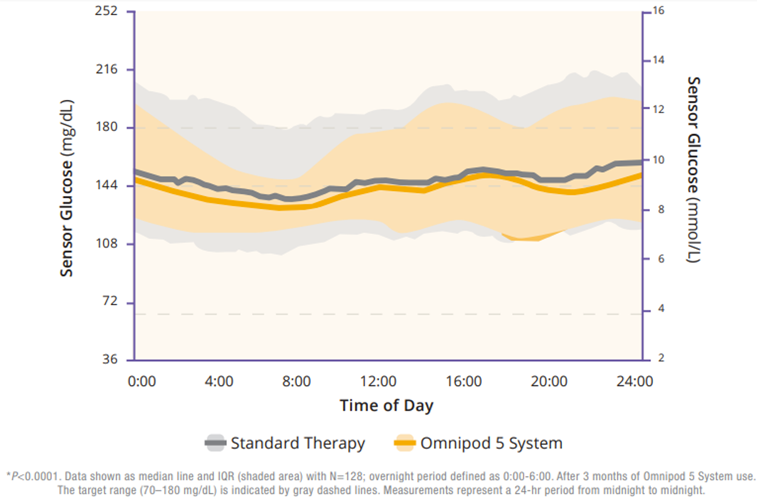
Regarding TIR, the adult/adolescent cohort achieved an improvement of 2.2 more hours per day of TIR as compared to standard therapy (Figure 2). Additionally, Omnipod 5 reduced time in hypoglycemia by 46% and time in hyperglycemia by 24% (Figure 3). Glycemic control overnight was also prominent with 78% TIR in this cohort (Figure 4).



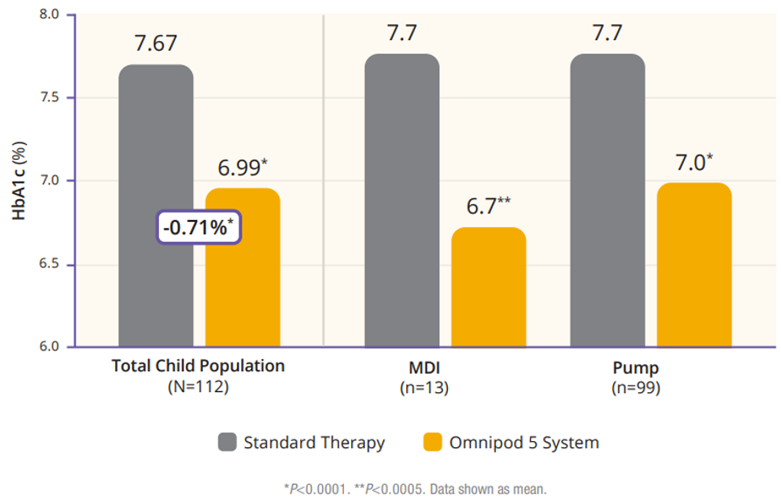
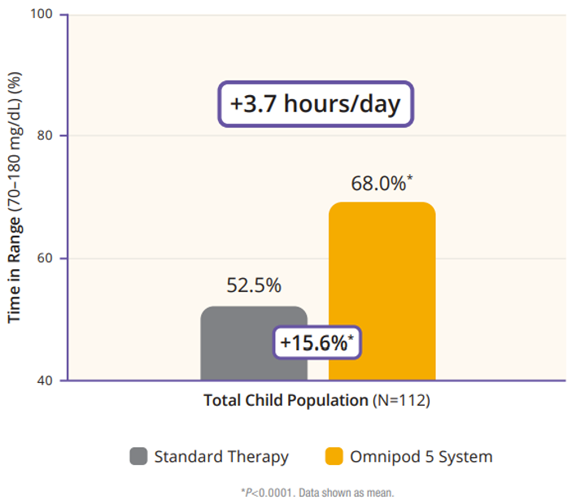
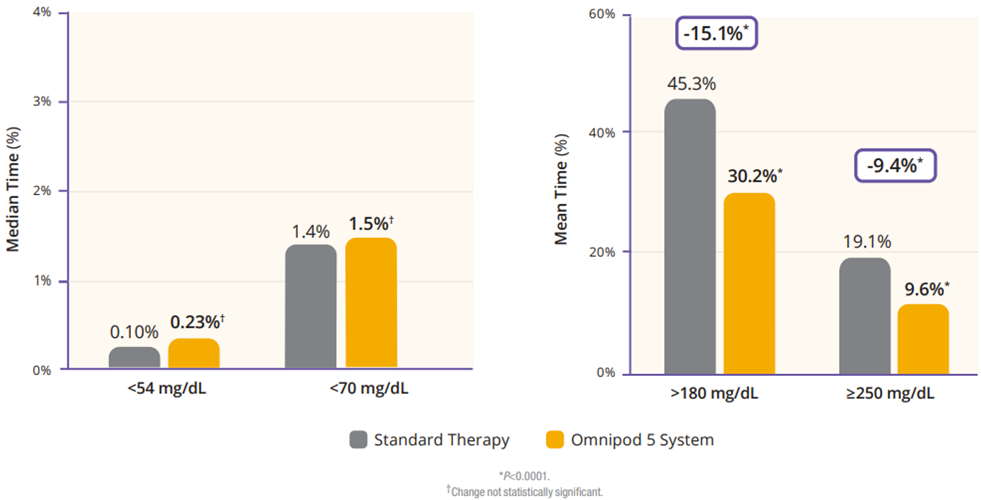
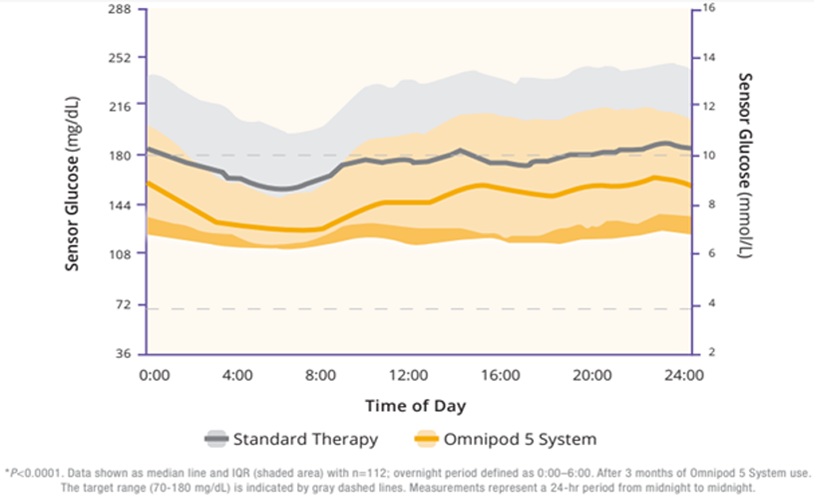
The children cohort experienced similarly favorable results. 53% achieved an HbA1C below 7%, compared to 23% at baseline (Figure 5). Their TIR was 68% which translated to an increase of 3.7 hours per day spent in range (Figure 6). This cohort already had a very low incidence of hypoglycemia with standard therapy. With Omnipod 5, that incidence rate remained low while rates of hyperglycemia were reduced by 33% (figure 7). Like the adult/adolescent group, children also experienced overnight TIR of 78% (Figure 8).




As for safety outcomes, there were 3 cases of severe hypoglycemia and 1 case of diabetic ketoacidosis (DKA) reported across both cohorts during Omnipod 5 use. The authors note that those “were below observed rates in type 1 diabetes and were not attributable to automated insulin delivery malfunction.”1
The study did have limitations, including the lack of a control group. The authors also point out that these “participants had relatively well-controlled baseline glycemic metrics, with many already using insulin pumps and glucose sensors, which may limit generalizability. However, the fact that there were improvements in this generally well-controlled group is encouraging.”1
Omnipod 5 was found to be safe and helped achieve significant improvements in the majority of participants’ HbA1C and TIR. To contextualize this first-of-its-kind tubeless AID system, the authors do note that “while differences in study design limit direct outcome comparisons, the results presented here indicate that the present system compares favorably with current commercially available devices.”
Finally, as a note of interest, it’s worth mentioning that while Brown et al. did not include very young children, a separate trial by Sherr et al. did study Omnipod 5 and children aged 2-5.9 years with similarly encouraging results.
Did you know Omnipod 5 is prescribed through the pharmacy, not through DME? This means many of your patients can start today.2
INS-OHS-11-2023-00174 v1.0